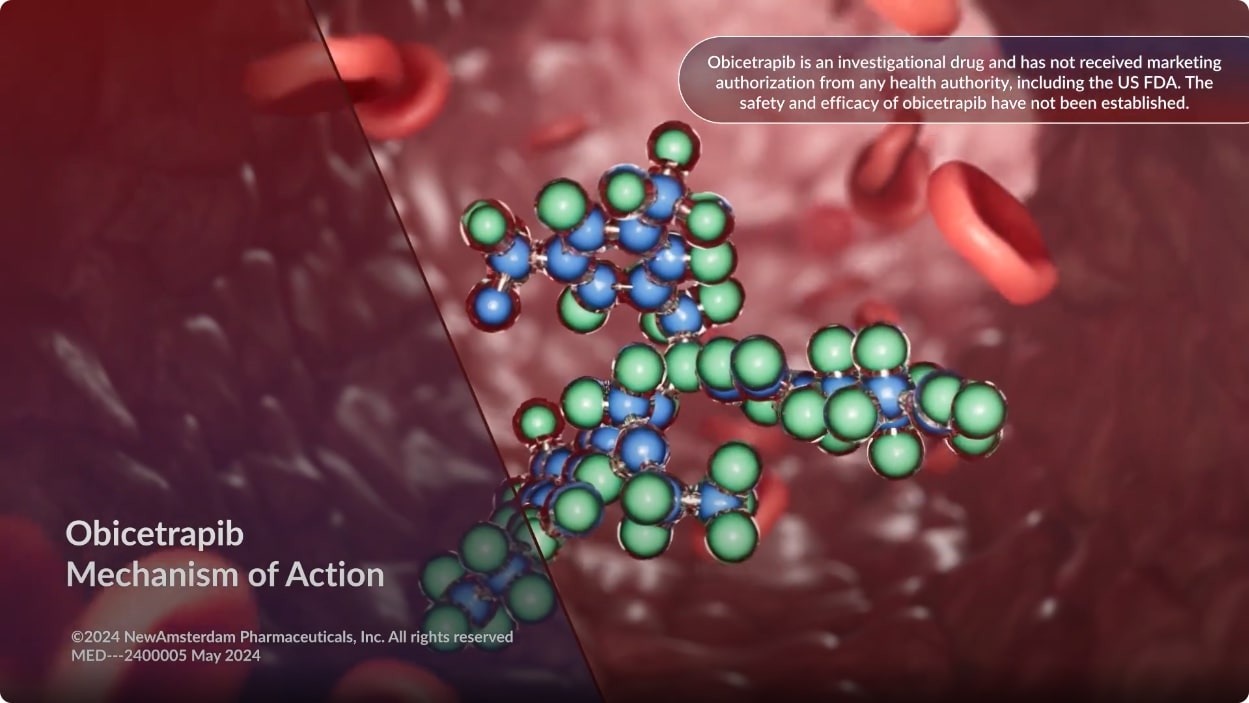

Obicetrapib
Obicetrapib is an investigational, highly selective cholesteryl ester transfer protein (CETP) inhibitor being studied for the treatment of elevated levels of low-density lipoprotein cholesterol (LDL-C).1
In preclinical studies, inhibiting CETP with obicetrapib reduced LDL-C levels by decreasing hepatic cholesterol, which led to an upregulation of LDL receptor expression and increased LDL clearance.2

Mechanism Of Action
Obicetrapib is currently being studied as a potential treatment to lower LDL-C as an
adjunct to maximally tolerated lipid-lowering therapies in patients with atherosclerotic
cardiovascular disease (ASCVD) or heterozygous familial hypercholesterolemia (HeFH) in
three pivotal Phase 3 trials: BROADWAY, BROOKLYN, and PREVAIL.3-5
Clinical programs
Registrational Studies
PHASE 1
PHASE 2
PHASE 3
Cardiovascular Disease
PREVAIL (NCT05202509) is a Phase 3, placebo-controlled, double-blind, randomized study in participants with ASCVD who are not adequately controlled despite maximally tolerated lipid-lowering therapy.5
Primary objective: Evaluate the effect of obicetrapib on the risk of major adverse cardiovascular events (MACE).
Secondary objectives: Evaluate the effect of obicetrapib on all-cause mortality, total cardiovascular events, new-onset diabetes, and change in LDL-C, non-HDL-C, and ApoB.
Completed Programs
PHASE 1
PHASE 2
PHASE 3
Cardiovascular Disease
BROADWAY (NCT05142722) is a Phase 3, placebo-controlled, double-blind, randomized study in patients with ASCVD and/or HeFH to evaluate the efficacy, safety, and tolerability of obicetrapib as an adjunct to diet and maximally tolerated lipid-lowering therapy.3
Primary objective: Evaluate the effect of obicetrapib on LDL-C levels.
Secondary objectives: Evaluate the effect of obicetrapib on apolipoprotein B (ApoB), apolipoprotein A1 (ApoA1), lipoprotein(a) (Lp(a)), high-density lipoprotein cholesterol (HDL-C), non-HDL-C, total cholesterol, and triglycerides.
Topline results: Observed an LS mean LDL-C reduction of 33% (p < 0.0001) compared with placebo at day 84 and observed a 21% reduction in major adverse cardiovascular events favoring obicetrapib at one year.
BROOKLYN (NCT05425745) is a Phase 3, placebo-controlled, double-blind, randomized study to evaluate the efficacy, safety, and tolerability of obicetrapib as an adjunct to diet and maximally tolerated lipid-lowering therapy in participants with a history of HeFH.4
Primary objective: Evaluate the effect of obicetrapib on LDL-C levels.
Secondary objectives: Evaluate the effect of obicetrapib on Lp(a), ApoB, and non-HDL-C.
Topline results: Observed an LS mean LDL-C reduction of 36.3% (p < 0.0001) compared with placebo at day 84, which was sustained at day 365 with an LS mean LDL-C reduction of 41.5% (p < 0.0001).
ROSE (NCT04753606) was designed as a placebo-controlled, double-blind, randomized, Phase 2, dose-finding study evaluating the efficacy, safety, and tolerability of obicetrapib used along with high-intensity statin therapy.6
Topline results: Patients treated for 8 weeks with 10 mg of obicetrapib achieved an LS mean LDL-C reduction of 40.1% (p < 0.0001) compared with placebo. Additionally, results found no serious adverse events (AEs) in the two obicetrapib cohorts, and two serious AEs in the placebo arm.

This Phase 2b study (NCT05421078) was a placebo-controlled, double-blind, randomized, dose-finding trial to evaluate the efficacy, safety, and tolerability of obicetrapib as an adjunct to stable statin therapy in Japanese patients.7
Topline results: Patients treated for 8 weeks with 10 mg of obicetrapib achieved an LS mean LDL-C reduction of 36.8% (p < 0.0001) compared with placebo.
Other Lipid-Related Conditions
This prespecified Alzheimer’s disease biomarker analysis in our BROADWAY (NCT05142722) trial evaluated the effect of our investigational CETP inhibitor, obicetrapib, in patients with cardiovascular disease who also had an elevated risk of Alzheimer’s disease. The analysis included a prespecified population of ApoE3/4 and ApoE4/4 carriers in whom plasma biomarkers of neurodegeneration were measured.
Topline Results: We observed lower absolute increases of plasma p-tau217 in both the full analysis set (p=0.0019) and in ApoE4 carriers (p=0.0215), and a significant reduction of 20.5% in plasma p-Tau217 compared to placebo (p=0.01) in ApoE4/4 carriers. Favorable trends were also observed across additional biomarkers including: p-Tau181, Aβ42:40 ratio, GFAP, NFL, p-Tau217/Aβ42:40 ratio.
This proof-of-concept Phase 2a trial (NCT05161715) was designed to explore the pharmacodynamics, cognitive effects, pharmacokinetics, safety, and tolerability of obicetrapib in early Alzheimer’s disease (AD) patients who are ApoE4 carriers.8
Topline Results: We observed reductions of 11% and 12% in 24- and 27-hydroxycholesterol in cerebrospinal fluid (CSF), respectively, indicating potential improvement of cholesterol metabolism in the brain, and observed 8% increase in Aβ42/40 ratio, a key biomarker of AD risk, suggesting improvement in disease pathology.


Expanded Access
1. Ford J, Lawson M, Fowler D, et al. Tolerability, pharmacokinetics and pharmacodynamics of TA-8995, a selective cholesteryl ester transfer protein (CETP) inhibitor, in healthy subjects. Br J Clin Pharmacol. 2014;78(3):498-508. doi:10.1111/bcp.12380 2. Inia J, Keijzer N, Worms N, et al. Obicetrapib alone and in combination with ezetimibe reduces non-HDL-cholesterol by enhanced LDL-receptor-mediated VLDL clearance and increased net fecal sterol excretion in apoe*3-leiden.cetp mice. Atherosclerosis. 2024;395,suppl1. 3. ClinicalTrials.gov. Randomized study to evaluate the effect of obicetrapib on top of maximum tolerated lipid-modifying therapies (BROADWAY). NCT05142722. Updated September 18, 2023. Accessed March 7, 2024. https://clinicaltrials.gov/study/NCT05142722 4. ClinicalTrials.gov. Evaluate the effect of obicetrapib in patients with HeFH on top of maximum tolerated lipid-modifying therapies. (BROOKLYN). NCT05425745. Last updated January 23, 2024. Accessed March 7, 2024. https://clinicaltrials.gov/study/NCT05425745 5. ClinicalTrials.gov. Cardiovascular outcome study to evaluate the effect of obicetrapib in patients with cardiovascular disease (PREVAIL). NCT05202509. Last updated March 7, 2024. Accessed May 5, 2024. https://clinicaltrials.gov/study/NCT05202509 6. ClinicalTrials.gov. Randomized study of obicetrapib as an adjunct to statin therapy (ROSE). NCT04753606. Last updated July 12, 2024. Accessed July 21, 2024. https://clinicaltrials.gov/study/NCT04753606 7. ClinicalTrials.gov. A Dose-Finding Study in Japanese Patients to Evaluate the Effect of Obicetrapib as an Adjunct to Stable Statin Therapy. Last updated September 27, 2024. Accessed September 27, 2024. https://clinicaltrials.gov/study/NCT05421078 8. ClinicalTrials.gov. Proof-of-concept, open-label study in patients with early Alzheimer’s disease. NCT05161715. Last updated May 4, 2024. Accessed July 21, 2024. https://clinicaltrials.gov/study/NCT05161715