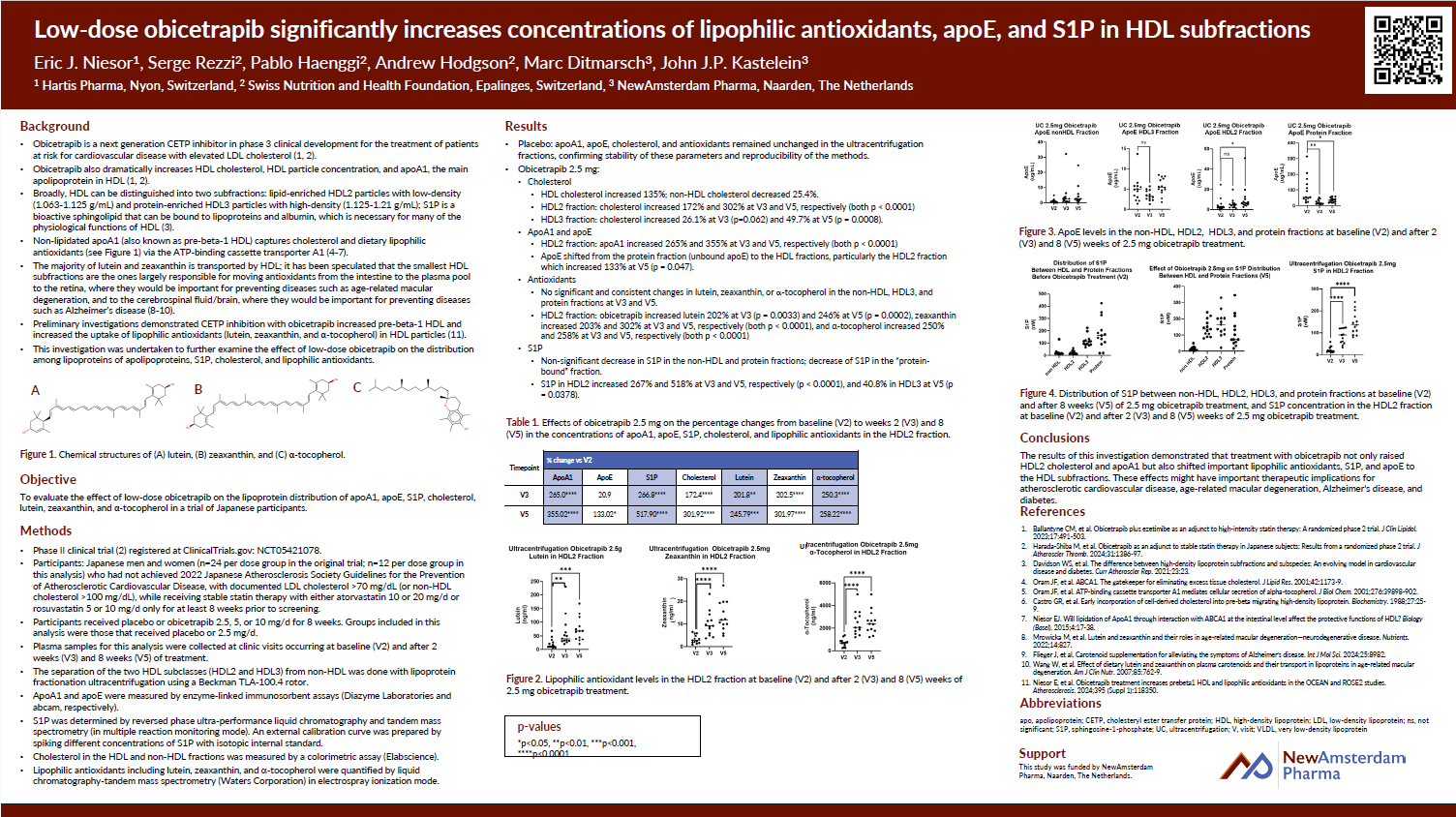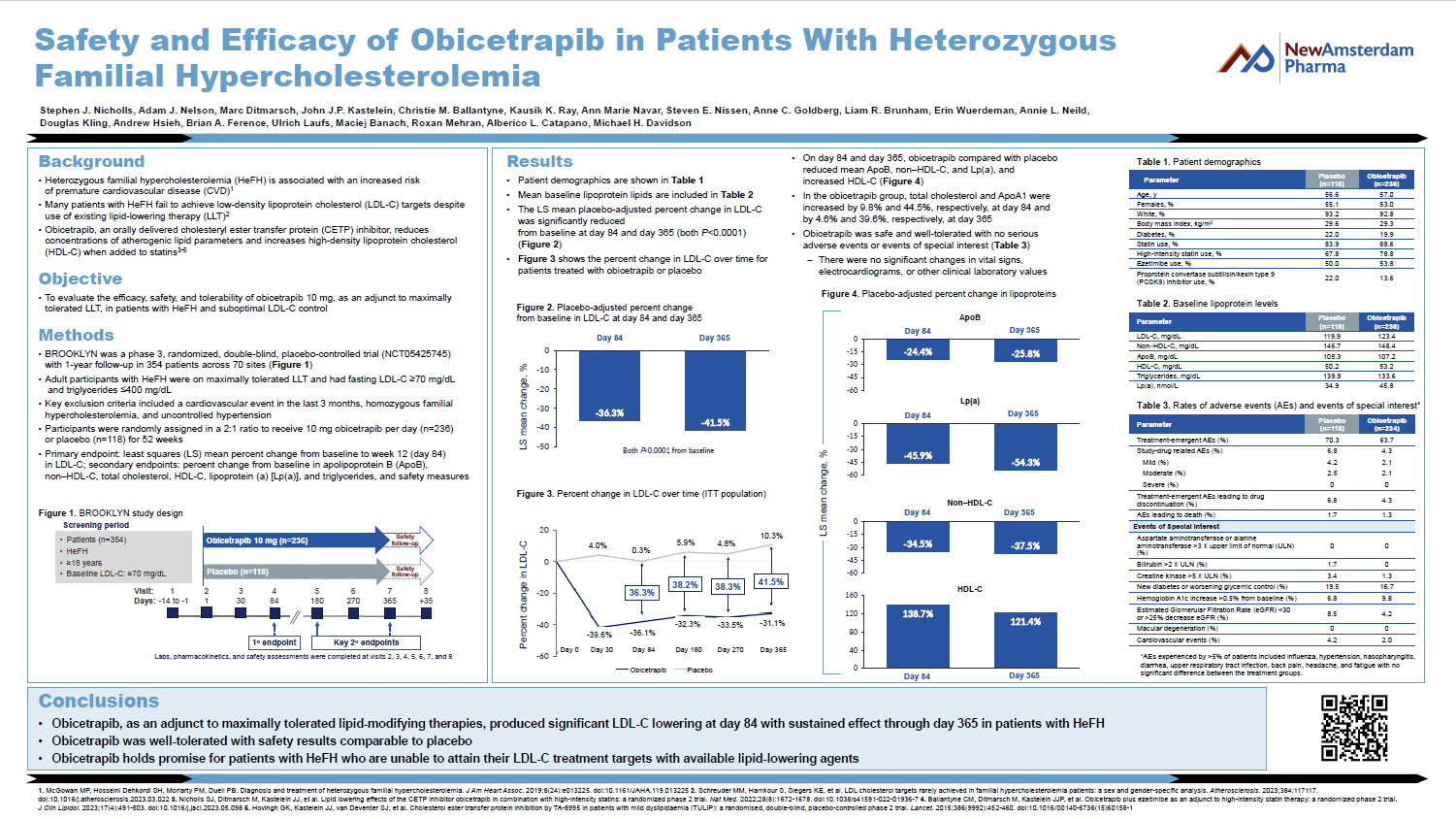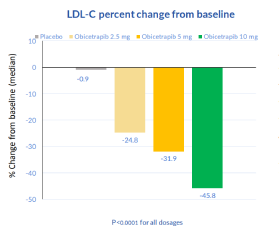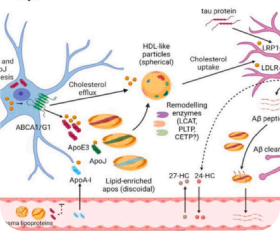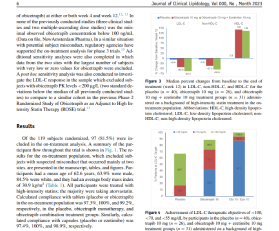

Scientific Publications and
Presentations
NewAmsterdam Pharma is dedicated to sharing knowledge and providing our science to
the medical, scientific, and patient communities through peer-reviewed presentations and
publications.


Medical Information for healthcare professionals licensed to practice in the United States and MSL request.